Oxycodone HCL Extended-release tablet 10 and 20 mg and 40 mg

Introduction
Faroxy®_ER tablets-manufactured by faran Shimi pharmaceutical company- contain oxycodone hydrochloride extended-release. Oxycodone belongs to a group of medicines called opioid analgesics. Faroxy®_ERis supplied in 10 mg and 20 mg extended-release tablets for oral administration. 10 tablets are packed in a blister and 6 blisters are packaged in one box with a leaflet.
Oxycodone is a semisynthetic pure opioid agonist whose principal therapeutic action is analgesia. Other pharmacological effects of oxycodone include anxiolysis, euphoria and feelings of relaxation. These effects are mediated by receptors (notably μ) in the central nervous system, Oxycodone binds to the μ-opioid receptor and activates the μ-opioid receptor, whereas it does not bind to the κ-opioid receptor and does not activate the κ-opioid receptor. Importantly, in human beings, oxycodone behaves as a μ-opioid receptor agonist producing analgesia, Oxycodone does not cause psychotomimetic effects, dysphoria, diuresis, or other effects typical for a κ-opioid agonist.
Indications
Faroxy®_ER tablets are an extended-release oral formulation of oxycodone hydrochloride, indicated for pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate in:
- Adults; and
- Opioid-tolerant pediatric patients 11 years of age and older who are already receiving and tolerate a minimum daily opioid dose of at least 20 mg oxycodone orally or its equivalent.
Important Information
Dosing
*All doses should be titrated to appropriate effect.
Dosing Adults:
- Initial: 10 mg every 12 hours.
- Dosage adjustment (titration): After initiation of oxycodone ER, adjust dose in increments (25% to 50%) no more frequently than every 1 to 2 days until desired pain control.
- Recommended maximum dose of Faroxy®_ER is 320 mg/ day.
* Patients may require rescue doses of an immediate-release analgesic during dose titration.
Dosing: Renal Impairment:
- CrCl ≥60 mL/minute: do not need to be adjusted.
- CrCl <60 mL/minute: doses of 33% to 50% of usual initial dosing have been recommended.
Dosing: Hepatic Impairment:
- Initial: Initiate oxycodone ER with 33% to 50% of the calculated recommended dose.
Dosing: Pediatric
**Note: Use only in pediatric patients ≥11 years of age who are already receiving opioid therapy for at least 5 consecutive days, tolerating a minimum daily opioid dose of at least 20 mg of oxycodone orally or its equivalent at least for the 2 days immediately prior to starting extended-release oxycodone tablets, and for which alternative treatment options are inadequate. Prior to initiation, all other around-the-clock opioid therapy must be discontinued.
- Initial: Initial dose: Children ≥11 years and Adolescents: Oral: Initial dose based on current opioid regimen dose; use the following conversion factor table and equation to convert the current opioid(s) daily dose to the extended-release oxycodone tablet daily dose.
- Initial dose of extended-release oxycodone tablets administered every 12 hours = (mg/day of current opioid regimen * conversion factor [1]) /2
[1] For conversion factors refer to Oral Opioid Analgesic Conversion factor Table
Dosing: Renal Impairment
- Doses of 33% to 50% of usual initial dosing have been recommended.
Dosing: Hepatic Impairment:
- Initial: One-third (1/3) to one-half (1/2) of the usual starting dose; carefully titrate dose to appropriate effect.
Oral Opioid Analgesic Conversion factor:
Calculating total daily doses of opioids is important to appropriately and effectively prescribe, manage, and taper opioid medications. The daily dose calculating of new opioid is as follows:
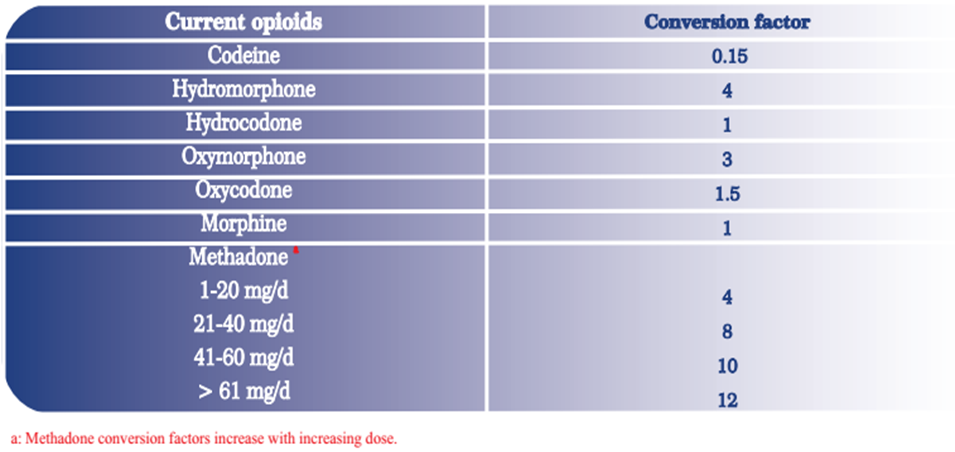

These dose conversion are estimated and cannot account for all individual differences in genetics and pharmacokinetics.
Discontinuation of therapy:
For discontinuing of chronic opioid therapy, the dose should be gradually tapered down. A universal tapering schedule for all patients hasn’t been established. Proposed schedules range from slow (e.g., 10% reductions per week) to rapid (e.g., 25% to 50% reduction every few days) (CDC 2015). Tapering schedules should be individualized to minimize opioid withdrawal while considering patient-specific goals and concerns as well as the pharmacokinetics of the opioid being tapered.
Administration
- Patients should have their dosage given on an around the-clock basis to prevent the reoccurrence of pain rather than treating the pain after it has occurred.
- Administer with or without food. Do not crush, break, chew, or dissolve the tablets.
- Faroxy®_ER tablets should be administered one at a time and each followed with water immediately after placing in the mouth.
- For oral use only, do not administer rectally.
Major Interactions (Risk X)[2]
- Azelastine (Nasal), Bromperidol, Conivaptan, Eluxadoline, Fusidic Acid (Systemic), Idelalisib, Opioids (mixed agonist/ antagonist), Orphenadrine, Oxomemazine, Paraldehyde, Thalidomide.[3]
[2] For other Interactions, See Uptodate, Acetaminophen codeine monograph
[3] For other Interactions, See UpToDate, oxycodone IR monograph
Pregnancy
- Faroxy®_ER is not commonly used to treat pain during labor and immediately postpartum (ACOG 209 2019) or chronic noncancer pain in pregnant women or those who may become pregnant.
Breast-Feeding
- Faroxy®_ER is present in breast milk. breastfeeding is not recommended during treatment with Faroxy®_ER.
Contraindications & Cautions
Contraindications
- Patients who are hypersensitive (e.g., anaphylaxis, angioedema) to oxycodone or any component of the formulation.
- Patients with circulatory shock and coma.
- Significant respiratory depression, acute or severe bronchial asthma
- Known or suspected paralytic ileus and gastrointestinal obstruction
Warnings/Precautions
Concerns related to adverse effects:
- CNS depression: May cause CNS depression, that may impair physical or mental abilities.
- Constipation: May cause constipation, which may be problematic in patients with unstable angina and patients’ post-myocardial infarction (MI). Consider preventive measures (e.g., stool softener, increased fiber) to reduce the potential for constipation.
- Hypotension: May cause severe hypotension (including orthostatic hypotension and syncope); use with caution in patients with hypovolemia, cardiovascular disease (including acute MI), or drugs that may exaggerate hypotensive effects (including phenothiazines or general anesthetics).
- Phenanthrene hypersensitivity: Use with caution in patients with hypersensitivity reactions to other phenanthrene-derivative opioid agonists (codeine, hydrocodone, hydromorphone, levorphanol, oxymorphone).
- Respiratory depression: Serious, life-threatening, or fatal respiratory depression may occur. Monitor closely for respiratory depression, especially during initiation or dose escalation.
Concerns related to adverse effects:
- CYP 3A4 interactions: Use with all CYP3A4 inhibitors may result in an increase in oxycodone plasma concentrations, which could increase or prolong adverse drug effects and may cause potentially fatal respiratory depression.
- Benzodiazepines or other CNS depressants: concomitant use of opioids with benzodiazepines or other CNS depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death.
Concerns related to adverse effects:
- Cachectic or debilitated patients: Use with caution in cachectic or debilitated patients; there is a greater potential for critical respiratory depression, even at therapeutic dosages.
- Elderly: Use with caution in the elderly; Consider the use of alternative nonopioid analgesics in these patients.
- Neonates: Neonatal withdrawal syndrome: Prolonged use of opioids during pregnancy can cause neonatal withdrawal syndrome, which may be life-threatening if not recognized and treated according to protocols developed by neonatology experts. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. Signs and symptoms include irritability, hyperactivity and abnormal sleep pattern, high pitched cry, tremor, vomiting, diarrhea, and failure to gain weight. Onset, duration and severity depend on the drug used, duration of use, maternal dose, and rate of drug elimination by the newborn.
- Abuse/misuse/diversion: Use exposes patients and other users to the risks of addiction, abuse, and misuse, potentially leading to overdose and death. Assess each patient’s risk prior to prescribing; monitor all patients regularly for development of these behaviors or conditions.
Side Effects
- Central nervous system: drowsiness, headache, dizziness
- Dermatologic: Pruritus
- Gastrointestinal: Nausea, constipation, vomiting
- Miscellaneous: Fever
References
- UpToDate 2019/ Oxycodone IR Drug Information
- FDA Label/ Oxycodone IR
- anesthesiology.pubs.asahq.org
- www.cdc.gov
